Use of Titanium Mesh in Implant Site Development for Restorative-Driven Implant Placement: Case Report. Part 1—Restorative Protocol for Single-Tooth Esthetic Zone Sites
Robert A. Levine, DDS, FCPP; Aleem Manji, DDS, MS; Joanie Faucher, DMD, MS; and Steven Present, DMD
Abstract:
Predictably augmenting alveolar bone is a significant challenge in implant site development. A restorative-driven approach to implant placement aids in achieving esthetic harmony in the final restoration. This case report demonstrates techniques for treating patients with significant bony defects using a titanium mesh scaffold along with prosthetic steps in molding and conditioning soft tissues with the provisional restoration and the fabrication of a custom impression coping. This case is part of a larger consecutive case series of 77 titanium mesh units in 62 patients treated in a private periodontal practice setting and restored in private restorative practices, which will be published subsequently. In this titanium mesh case series, 14 other cases (a total of 15) were treated in a similar fashion as described in this case report in the category of “single implant placement in the maxillary anterior region.” This case illustrates the primary advantages of the use of titanium mesh in such clinical situations.
The ability to predictably augment alveolar bone in both a horizontal and vertical dimension is one of the most challenging surgical procedures in implant site development.1 It is also one of the key determinants in obtaining a long-term esthetic and functional result. The ITI Treatment Guide states, “An esthetic implant prosthesis is defined as one that is in harmony with the peri-oral facial structures of the patient. The esthetic peri-implant tissues, including health, height, volume, color, and contours, must be in harmony with the surrounding dentition. The restoration should imitate the natural appearance of the missing dental unit(s) in color, form, texture, size, and optical properties.”2 A restorative-driven approach to implant placement aids in achieving this esthetic harmony in the final restoration.3-6
One of the risk factors evaluated presurgically in attaining an esthetic final result is inadequate bone volume in three dimensions. In these cases the placement of a dental implant becomes more complicated with less predictability, and the implant is frequently placed in a poor, more palatal or apical prosthetic position; this does not allow for the achievement of long-term health, function, and esthetics. Studies have shown that the surgeon’s goal is a final osteotomy with ≥ 2 mm of bone to the facial of the implant osteotomy with corresponding thick tissue and an adequate band of keratinized gingiva.7-10 This becomes very important in esthetically demanding areas as in a patient’s esthetic zone.
Implant treatment in the esthetic zone is a challenging procedure and classified as advanced or complex according to the Straightforward, Advanced, and Complex (SAC) Classification.11 It requires comprehensive preoperative planning and precise surgical execution based on a restorative-driven approach.3-5,12,13 Most patients present with multiple esthetic risk factors and often have high expectations. Dental implant treatment in the esthetic zone thus requires immaculate execution by skillful clinicians as a prerequisite to attempting this procedure. If esthetic complications occur, they are usually difficult or impossible to manage.13-16 Consequently, the prevention of esthetic complications should be a primary objective. Therefore, a conservative treatment approach is recommended to facilitate successful outcomes with high predictability and a low risk of complications.14,15
Pretreatment Assessment
The SAC Classification was developed to aid in clinical decision-making for the benefit of the patient and to help avoid complications based on the experience level of the clinician and the potential difficulty of the treated implant site. The SAC Classification System has both restorative and surgical categories that use a normative classification system, which can be influenced by modifying factors based on individual clinical situations. One area that can influence this classification—both from a surgical and restorative perspective—is found in the International Team for Implantology (ITI) esthetic risk assessment (ERA) analysis. The ERA is a pretreatment assessment tool that uses clinical precursors to determine the risk of achieving an esthetic result based on known surgical and restorative approaches in given clinical situations.2,4,5
Esthetic risk factors should be addressed with the patient before the initiation of treatment to avoid any post-treatment misunderstandings that may result from unmet high expectations. The clinician can best avoid potential post-treatment complications and a dissatisfied patient by gathering information with the patient chairside during the initial consultation visit and sharing it with him or her using aids such as the ERA form. This is also an excellent team (ie, surgeon, restorative dentist, dental laboratory, and patient) communication tool that can be used in all esthetic cases to help achieve esthetic goals.5 As part of the ERA tool, horizontal (medium risk) and vertical (high risk) bony deficiency of the planned implant site is evaluated at the initial consultation visit. Minor amounts of horizontal augmentation can be accomplished readily with a wide variety of treatment modalities. However, situations requiring greater amounts of bone augmentation are more challenging. Distraction osteogenesis, autogenous onlay block grafts, ridge splitting, and tenting screws with barrier membranes (with or without titanium struts) have been described in the literature as techniques capable of producing significant amounts of guided bone regeneration (GBR). However, with each of these techniques there are concerns related to predictability, patient morbidity, or postoperative complications.1,17 The therapeutic goal is to provide the patient the best evidenced-based therapy with the least risk of patient morbidity.
Titanium Mesh Scaffold
The use of epithelium-excluding barrier membranes for the purposes of GBR has been demonstrated in the literature to be a successful treatment approach. However, complications related to early exposure and early removal of the barrier membrane make this technique less predictable when significant regeneration is required. Boyne introduced the concept of a titanium mesh scaffold as an alternative to traditional barrier membranes.18 The advantage purported at the time was the ability to offer significantly increased and continual space maintenance during the healing phase to allow bone regeneration to occur with fewer concerns about failure if the mesh became exposed. Since that time, numerous studies have reported the success of this technique in achieving a significant amount of osseous regeneration in implant site development procedures.19-27
The following case report demonstrates the techniques used by the primary author to treat patients with significant bony defects using a titanium mesh scaffold along with the prosthetic steps in molding and conditioning the soft tissues with the provisional restoration and the fabrication of a custom impression coping with the restorative dentist. This case is part of a larger consecutive case series of 77 titanium meshes in 62 patients treated in the primary author’s private periodontal practice setting and restored in private restorative practices, which will be published subsequently. As part of this titanium mesh case series, 14 other cases (a total of 15) were treated in a similar fashion as described in this case report in the category of “single implant placement in the maxillary anterior region.” A total of 55 titanium meshes were used in the maxillary arch and 22 in the mandibular arch.
Case Report
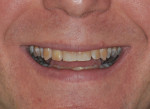
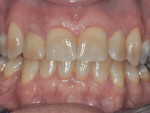
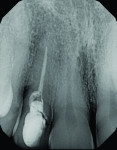
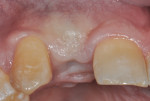
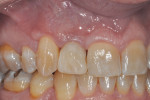
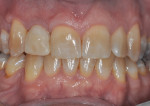
The patient, a 49-year-old white man, was referred for extraction of tooth No. 7, which had been diagnosed with a nonrestorable root fracture (Figure 1 through Figure 4). Esthetically, the patient presented with a low lip line, a low scalloped thick gingival biotype, and rectangular shaped teeth. The ERA was reviewed chairside with the patient after full-mouth digital photographs were taken and a low-medium esthetic risk was diagnosed. The patient was a controlled type 2 diabetic and a nonsmoker (American Society of Anesthesiologists [ASA] 2). Probing depths proximally to tooth No. 7 were 5 mm to 6 mm, and midbuccal/palatal probing depths were 5 mm with a 2-degree mobility of this hopeless tooth.
The tooth was extracted without complication via a minimally traumatic approach after full-thickness flaps were reflected and using a Piezosurgery® device (Piezosurgery Incorporated, www.piezosurgery.us). The Piezosurgery was used after flap reflection in such a manner as to remove tooth structure while avoiding overheating the bone with copious amounts of sterile water irrigation (using the Piezosurgery EX1 and EX2 inserts). A trough was created around the tooth at the expense of tooth structure, which aided in the easy removal of the tooth with small elevators followed by gentle pressure with forceps. The socket was thoroughly debrided using the following protocol to help in disinfection of the socket interior: 1) small surgical curettes; 2) Piezosurgery (using the OT4 insert); 3) irrigation for 1 minute with 10% povidone-iodine; 4) irrigation for 1 minute with sterile water.
Because of bone loss circumferentially and, especially, midbuccal, an immediate implant was contraindicated. A CollaPlug® (Zimmer Dental, www.zimmerdental.com) was placed in the socket for hemostasis and the area was sutured with 6-0 plain gut with a P-3 needle. A transitional partial denture was delivered and adjusted on the day of surgery.
GBR Procedure
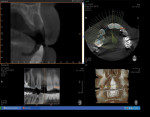
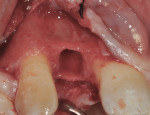
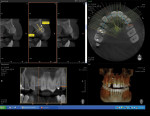
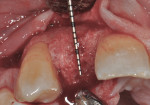
At 3 months post-extraction, a clinical examination and a cone-beam computed tomography (CBCT) scan were completed, revealing a significant loss of vertical bone height (Figure 5). The site was re-entered to perform a GBR procedure using a titanium mesh scaffold. The area was anesthetized, full-thickness buccal and palatal flaps were reflected with a disto-buccal vertical-releasing incision at tooth No. 6 (for good access to the bony defect), and the buccal flap was then sutured to the buccal mucosa to reduce trauma to the flap and for increased visualization. Figure 6 shows the residual defect, which had an intact facial and palatal plate; however, both plates were approximately 3-mm apical of the desired height. In addition, there was noted attachment loss with corresponding bone loss on the distal aspect of tooth No. 8.
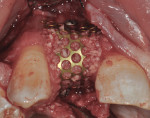
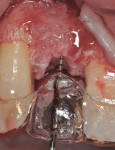
The area was debrided to remove all soft-tissue fragments, and numerous intramarrow penetrations were performed with the Piezosurgery using the OP5 insert. The residual defect was then grafted with a combination of freeze-dried bone allograft (Puros®, Zimmer Dental) and recombinant human platelet-derived growth factor (GEM 21S®, Ostheohealth Co., www.osteohealth.com). A simultaneous guided tissue regeneration (GTR) procedure on the distal aspect of tooth No. 8 was also completed with these materials. The graft was covered with a 0.3-mm titanium mesh (Synthes, Inc., www.synthes.com), stabilizing the buccal first with a 3-mm stabilizing screw (Synthes, Inc.) to create a buccal wall to pack the bone graft. The titanium mesh needs to be kept at least 1.5 mm to 2 mm away from adjacent tooth surfaces when fully secured to prevent bacterial penetration from the tooth surface under the titanium mesh during healing. A second 3-mm stabilizing screw was used to secure the titanium mesh in place on the palatal aspect to prevent any micromovement of the mesh, which has been shown to create soft, not hard tissue. All sharp corners of the mesh were tucked downward with a small amalgam plugger to prevent tissue fenestration. This additionally helped secure the mesh in place (Figure 7).
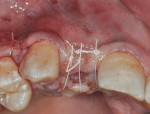
A piece of CollaTape® absorbable collagen (Zimmer Dental) was customized and then soaked in GEM 21S and placed over the titanium mesh and up against the distal of tooth No. 8 to help in graft containment and delivery of the GEM 21S-soaked bone graft to the distal root surface of No. 8 and the overlying flap. The flaps were sutured to obtain tension-free primary closure using 4-0 Cytoplast™ polytetrafluoroethylene (PTFE) sutures (Osteogenics Biomedical, www.osteogenics.com) and 6-0 plain gut. The tension-free closure was attained by completing a deep buccal periosteal-releasing incision before suturing (Figure 8). The temporary partial denture was relieved to not exert any pressure on the graft area during the initial healing period.
Implant Procedure
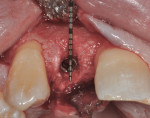
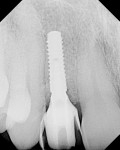
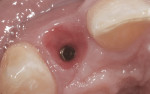
After 6 months of healing, a CBCT scan was taken of the surgical site (Figure 9). The scan showed > 7 mm of thickness of bone in a bucco-palatal direction with a significant vertical height gained compared with the pretreatment CBCT scan in Figure 5. After an additional month of healing (Figure 10), the patient returned for the surgical implant procedure. The area was anesthetized with local anesthetic, and buccal and lingual full-thickness flaps were reflected with a disto-buccal vertical release to tooth No. 6. It was confirmed with a periodontal probe that there was > 7 mm of width at the crest (Figure 11), with the crest positioned about 2 mm to 3 mm more coronal when compared with the original defect (Figure 5). In addition, GTR was achieved on the distal of tooth No. 8. In fact, it was necessary to remove midbuccal bone with the aid of an anatomically correct surgical guide template with a high-speed #8 round bur (bone scalloping) to place the implant at an ideal depth to facilitate proper emergence profile for the final crown. For a bone-level (BL) implant, approximately 4 mm of space is needed from the midbuccal of the surgical guide to the osseous crest (Figure 12). A Straumann® 3.3-mm x 12-mm NC SLActive implant (Straumann USA LLC, www.straumann.com) was placed with the aid of the anatomically correct surgical guide template and achieved excellent primary stability in type 3 bone.
As shown in Figure 13, a measurement of 3 mm of bone was buccal to the implant. This can be compared with Figure 6, in which a significant reduction of the buccal concavity resulting from the GBR procedure was evident. This is critical because all of the patients in this current study that had < 1.5 mm of bone to the facial of the osteotomy were grafted with a slowly resorbing xenograft (particle size 0.25 mm to 1 mm) (Bio-Oss®, Geistlich Biomaterials, www.geistlichonline.com) for “contour augmentation” and covered with a collagen membrane (Bio-Gide®, Geistlich Biomaterials) to aid in hard- and soft-tissue maintenance over time.28 A bottleneck healing abutment was placed, and the area was sutured with 6-0 polypropylene sutures. This initial healing abutment would be removed at 8 weeks and replaced by a tapered healing abutment at a “stretch the tissue visit” to provide adequate subgingival room to start the provisionalization phase. The implant was also torque-tested to 35 Ncm to confirm bone healing at the 8-week postoperative visit, which is the time that is generally recommended for bone-to-implant healing in a regenerated bone graft site.
Prosthetic Phase
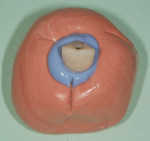
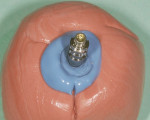
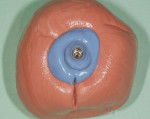
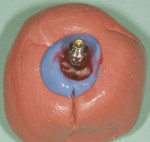
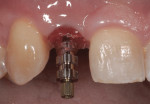
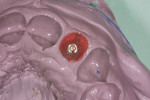
The patient presented for the final restoration to be fabricated on the previously placed, fully integrated bone-level 3.3-mm NC SLActive implant replacing the right maxillary lateral incisor. Since this was a healed site and an anatomically correct surgical guide was used for restorative-driven implant placement, a screw-retained provisional crown was fabricated with the anticipated ideal contours in order to develop the transition zone and sculpt the gingival margins and papillas to mimic the contours of the contralateral incisor. The patient was recalled to the restorative office every 2 weeks to re-evaluate the gingival and provisional contours and to make any necessary adjustments (ie, addition and/or subtraction of acrylic) to the provisional crown. After 8 weeks, the site was ready for the final impression (Figure 14 through Figure 16). To reproduce the final development of the transition zone in the impression, a custom impression coping was fabricated (Figure 17 through Figure 23). The final restoration was coated with 1% chlorhexidine gel (Corsodyl® 1%, GlaxoSmithKline, www.corsodyl.co.uk), and the screw was torqued to 35 Ncm, covered with Teflon® tape (DuPont, www.dupont.com), and sealed with composite resin.
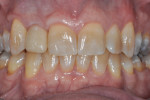
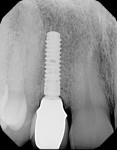
Figure 24 and Figure 25 show the final case and digital periapical radiograph, respectively, at 1-year post-crown. Excellent soft- and hard-tissue healing with 100% papilla fill for the distal interproximal area and 50% in the mesial interproximal area were achieved. Additional papilla fill can be anticipated over time.9
Discussion
As observed in this case report, significant amounts of bone regeneration were achieved through use of titanium mesh. The patient was successfully rehabilitated with a dental implant, allowing him to be restored to health, function, and esthetics in his maxillary lateral incisor area.
The case presented is part of a large, consecutive series study on the use of a titanium mesh scaffold for GBR. To date, 77 distinct sites of titanium mesh have been placed in 62 patients. Of the 121 implants that have been treatment planned, all have been placed successfully except one because of early mesh exposure and loss of the mesh and graft. Only one early loss of an implant was observed after placement using the described titanium mesh technique (99.2% survival to date). Preliminary data shows average gains in horizontal ridge width of 4.98 mm and vertical gains of 3.1 mm in those cases where such augmentation procedures were required.
Several trends have also been noted to date during the course of this larger study of 77 titanium mesh units. First, exposure of the mesh generally shows no signs of infection and does not appear to compromise the success rate of the procedure. A total of 19 exposures (12 early [ie, < 3 months] and 7 late [ie, > 3 months]) were recorded. The majority (11/19) were in thin tissue biotypes. Upon titanium mesh removal in the exposed titanium mesh cases, increased thickness of the pseudo-periosteum layer under the mesh was observed and the bone quality appeared to be lower in the area of exposure. Only one complete graft failure was recorded in an early exposure case, which was re-treated successfully. Second, the use of biologic modifiers (such as platelet-rich growth factor, Gem 21S, and Osteocel® [Ace Surgical Supply Co., Inc., www.acesurgical.com]) appears to enhance the results of augmentation and lowers the rate of exposures. Third, of the 121 implants placed, 63 required additional bone grafting for “contour augmentation” (52%) with slowly resorbing bovine bone and a collagen membrane to provide additional thickness to the facial and to help ensure a long-term esthetic result with its corresponding soft- and hard-tissue maintenance.14,28
Conclusions
The main advantages of the titanium mesh are:
• It enables predictable space maintenance for GBR for both horizontal and vertical ridge reconstruction.
• It yields low patient morbidity compared to autogenous block grafts.
• All size defects are amenable to the procedure.
• Use of the ERA analysis is recommended as a pretreatment assessment tool that uses clinical precursors to determine the risk of achieving an esthetic result.2,4,5 Esthetic risk factors should be addressed directly with the patient before the initiation of treatment to avoid any post-treatment misunderstandings that may result from unmet high expectations.2-5
• The use of an anatomically correct surgical guide provides for a restorative-driven outcome. This enables the final restoration to be a desired screw-retained crown, thus eliminating the possible biologic sequela of cementation associated with subgingival cement remnants and its associated peri-implant diseases.3-5,29-32
(Editor's Note: Part 2 of this series, "Surgical Protocol for Single-Tooth Esthetic Zone Sites," appears in the May 2014 issue of Compendium.)
References
1. Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants. 2009;24(suppl):237-259.
2. Martin W, Morton C, Buser D. Pre-operative analysis and prosthetic treatment planning in esthetic implant dentistry. In: Buser D, Belser U, Wismeijer D, eds. ITI Treatment Guide: Implant Therapy in the Esthetic Zone for Single-Tooth Replacements. Hanover Park, IL: Quintessence Publishing Co. Inc.; 2007:9-24.
3. Buser D, Martin W, Belser UC. Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Implants. 2004;19(suppl):43-61.
4. Levine RA, Nack G. Team treatment planning for the replacement of esthetic zone teeth with dental implants. Compend Contin Educ Dent. 2011;32(4):44-50.
5. Levine R, Martin W. Esthetic risk assessment in implant dentistry. Inside Dentistry. 2012;8(8):66-71.
6. Belser UC, Schmid B, Higginbottom F, Buser D. Outcome analysis of implant restorations located in the anterior maxilla: a review of the recent literature. Int J Oral Maxillofac Implants. 2004;19(suppl):30-42.
7. Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restortive Dent. 2005;25(2):113-119.
8. Spray JR, Black CG, Morris HF, Ochi S. The influence of bone thickness on facial marginal bone response: stage 1 placement through stage 2 uncovering. Ann Periodontol. 2000;5(1):119-128.
9. Kan JY, Rungcharassaeng K, Lozada JL, Zimmerman G. Facial gingival tissue stability following immediate placement and provisionalization of maxillary anterior single implants: a 2- to 8-year follow-up. Int J Oral Maxillofac Implants. 2011;26(1):179-187.
10. Alpert A. A rationale for attached gingiva at the soft-tissue/implant interface: esthetic and functional dictates. Compend Contin Educ Dent. 1994;15(3):356, 358, 360-362.
11. Dawson A, Chen S, eds. The SAC Classification in Implant Dentistry. Berlin, Germany: Quintessence Publishing Co, Ltd; 2009:8.
12. Buser D, Belser U, Wismeijer D, eds. ITI Treatment Guide: Implant Therapy in the Esthetic Zone for Single-Tooth Replacements. Hanover Park, IL: Quintessence Publishing Co. Inc.; 2007:1-24.
13. Evans CD, Chen ST. Esthetic outcomes of immediate implant placements. Clin Oral Implants Res. 2008;19(1):73-80.
14. Levine RA, Huynh-Ba, Cochran DL. Soft tissue augmentation procedures for mucogingival defects in esthetic sites. Int J Oral Maxillofac Implants. 2014. In press.
15. Morton D, Chen ST, Martin WC, et al. Consensus statements and recommended clinical procedures regarding optimizing esthetic outcomes in implant dentistry. Int J Oral Maxillofac Implants. 2014. In press.
16. Chen ST, Buser D. Clinical and esthetic outcomes of implants placed in postextraction sites. Int J Oral Maxillofac Implants. 2009;24(suppl):186-217.
17. Levine R. Implant site preparation: horizontal ridge augmentation using particulate allograft and the principles of guided bone regeneration. In: Sonick M, Hwang D, eds. Implant Site Development. 1st ed. Hoboken, NJ: Wiley-Blackwell; 2011:179-201.
18. Boyne PJ, Cole MD, Stringer D, Shafqat JP. A technique for osseous restoration of deficient edentulous maxillary ridges. J Oral Maxillofac Surg. 1985;43(2):87-91.
19. von Arx T, Kurt B. Implant placement and simultaneous peri-implant bone grafting using a micro titanium mesh for graft stabilization. Int J Periodontics Restorative Dent. 1998;18(2):117-127.
20. von Arx, T, Kurt B. Implant placement and simultaneous ridge augmentation using autogenous bone and a micro titanium mesh: a prospective clinical study with 20 implants. Clin Oral Implants Res. 1999;10(1):24-33.
21. Artzi Z, Dayan D, Alpern Y, Nemcovsky CE. Vertical ridge augmentation using xenogenic material supported by a configured titanium mesh: clinicohistopathologic and histochemical study. Int J Oral Maxillofac Implants. 2003;18(3):400-406.
22. Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006;32(5):237-247.
23. Longoni S, Sartori M, Apruzzese D, Baldoni M. Preliminary clinical and histologic evaluation of a bilateral 3-dimensional reconstruction in an atrophic mandible: a case report. Int J Oral Maxillofac Implants. 2007;22(3):478-483.
24. Roccuzzo M, Ramieri G, Bunino M, Berrone S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: a controlled clinical trial. Clin Oral Implants Res. 2007;18(3):286-294.
25. Louis PJ, Gutta R, Said-Al-Naief N, Bartolucci AA. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J Oral Maxillofac Surg. 2008;66(2):235-245.
26. Misch CM. Bone augmentation of the atrophic posterior mandible for dental implants using rhBMP-2 and titanium mesh: clinical technique and early results. Int J Periodontics Restorative Dent. 2011;31(6):581-589.
27. Her S, Kang T, Fien MJ. Titanium mesh as an alternative to a membrane for ridge augmentation. J Oral Maxillofac Surg. 2012;70(4):803-810.
28. Buser D, Wittneben J, Bornstein MM, et al. Stability of contour augmentation and esthetic outcomes of implant-supported single crowns in the esthetic zone: 3-year results of a prospective study with early implant placement postextraction. J Periodontol. 2011;82(3):342-349.
29. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388-1392.
30. Linkevicius T, Vindasiute E, Puisys A, Peciuliene V. The influence of margin location on the amount of undetected cement excess after delivery of cement-retained implant restorations. Clin Oral Implants Res. 2011;22(12):1379-1384.
31. Levine RA, Present S, Wilson TG. Complications with excess cement and dental implants. Part 1: diagnosis, recommendations & treatment of 7 clinical cases. Implant Realities. 2013. In press.
32. Present S, Levine RA. Techniques to control or avoid cement around implant retained restorations. Compend Contin Educ Dent. 2013;34(6);432-437.
About the Authors
Robert A. Levine, DDS, FCPP
Clinical Professor in Periodontology and Implantology, Kornberg School of Dentistry, Temple University, Philadelphia, Pennsylvania; Private Practice, Pennsylvania Center for Dental Implants and Periodontics, Philadelphia, Pennsylvania; Diplomate, American Board of Periodontology; Fellow, International Team for Implantology, Basel, Switzerland
Aleem Manji, DDS, MS
Former Postgraduate Resident in Periodontology and Implantology, Kornberg School of Dentistry, Temple University, Philadelphia, Pennsylvania; Private Practice, Toronto, Canada; Diplomate, American Board of Periodontology; Fellow, Royal College of Dentists of Canada
Joanie Faucher, DMD, MS
Professor, Department of Periodontology, Laval University, Quebec City, Canada; Former Postgraduate Resident in Periodontology and Implantology, Kornberg School of Dentistry, Temple University, Philadelphia, Pennsylvania; Private Practice, Quebec, Canada; Diplomate, American Board of Periodontology; Fellow, Royal College of Dentists of Canada
Steven Present, DMD
Clinical Associate Professor, Kornberg School of Dentistry, Temple University, Philadelphia, Pennsylvania; Private Practice, North Wales, Pennsylvania; Fellow, International Team for Implantology, Basel, Switzerland