You must be signed in to read the rest of this article.
Registration on AEGIS Dental Network is free. Sign up today!
Forgot your password? Click Here!
The Ribroast Technique™: An Alternative Method to Stabilize a Resorbable Collagen Membrane for Guided Bone Regeneration
Matthew Fien, DDS; Israel Puterman, DMD, MSD; Juan Mesquida, DDS; Guillermo Bauza, PhD; and Ignacio Ginebreda, DDS
Abstract: Stabilization of the graft-membrane complex during guided bone regeneration is a critically important aspect of implant dentistry. Several articles in the dental literature have introduced the utilization of periosteal biting stabilization sutures, rather than fixation screws and pins, to stabilize a bioabsorbable collagen membrane. This article reviews the concept of stabilization using sutures in periodontal regeneration and describes an alternative method, the Ribroast technique™, to stabilize a bioabsorbable membrane, whereby single periosteal biting horizontal mattress sutures are placed along the length of the defect to achieve sufficient stabilization. Two cases are presented that highlight the utility of this technique. Guidelines and limitations for the use of periosteal biting sutures are also discussed along with considerations and protocols that may be useful for improving treatment outcomes.
Guided bone regeneration (GBR) involves the use of a cell-occlusive barrier membrane to confine a particulate bone graft to a defect, creating a space where a blood clot can form and bone formation can occur.1-3 Regardless of the type of membrane used, stabilization of the graft-membrane complex has been identified as a key requirement for optimal regeneration.4 This is because substantial stabilization is required to minimize micromovement, which can result in the formation of granulation tissue as opposed to new bone formation.5-7 In addition, optimal stabilization allows for a greater volume of particulate bone to be placed within the defect to maximize space maintenance.
When utilizing a nonresorbable membrane, sufficient long-term stabilization of the rigid device must be provided, and therefore, the use of screws and membrane pins are recommended.8-10 Membrane fixation screws and pins can be challenging to place for practical reasons such as the presence of dense cortical bone and difficult access at various locations in the oral cavity. Although the use of pilot holes in the dense cortical bone, strong pins and screws that do not bend, and improved protocols have led to better treatment outcomes with the combination of fixation pins and resorbable collagen membranes,10,11 the increased technical demands of the procedure are apparent. Moreover, the composition and handling properties of collagen membranes vary, and many bioabsorbable collagen membranes cannot be stabilized with screws and pins without tearing. For these reasons, multiple authors have described techniques to stabilize a collagen membrane utilizing periosteal biting sutures as opposed to pins and screws.12,13 In 2016, Urban et al described the use of vertical mattress periosteal sutures,14 and Neiva et al has shown successful results with the Lasso technique, whereby a continuous periosteal biting suture is used to stabilize the graft-membrane complex.15,16
This article describes the Ribroast technique™ (trademarked by the author MF), which is a modification to these previously described techniques for membrane stabilization. It is an alternative option to stabilize the graft-membrane complex. Two cases are presented to illustrate the utility of the technique.
Technique Description
After local anesthesia is administered, a 15c blade is used to place a crestal incision over the edentulous ridge, bisecting the band of keratinized tissue when possible. A vertical releasing incision one or two teeth mesial or distal to the defect, extending past the mucogingival junction (MGJ), can be placed to improve surgical access to the depth of the defect as well as for access to bite the apical periosteum within the internal aspect of the buccal flap while performing membrane stabilization with sutures.
After placement of the incisions, a full-thickness mucoperiosteal flap is elevated, beginning on one corner of the incision and extending 3 mm to 5 mm beyond the margin of the defect. The site is further prepared by means of intra-marrow penetrations to expose the medullary blood supply; the authors also recommend obtaining autogenous bone via cortical scraping at this time.17
Before adapting the bone graft and membrane to the defect and attaining subsequent stabilization, a periosteal dissection must be performed to release flap tension, and a secondary internal flap is created that can be used to anchor the sutures on the buccal side. The authors recommend the technique best described by Ronda and Stacci.18 It involves the placement of a 1-mm deep periosteal incision in a single plane along the length of the flap just apical to the MGJ. After placement of this initial shallow incision, a micro-elevator or other similar instrument is used to stretch within this incision line while grasping the flap margin with sufficient tension. This releases tension in the flap for eventual dual-layered primary closure to minimize the risk of loss of biomaterials and postoperative complications such as incision line opening.4,19 This maneuver also creates a distinct internal flap that is no longer intimately associated with the buccal flap coronal to the releasing incision but, rather, with the bone at the most apical extent of the flap preparation, where the periosteum has not been reflected from the alveolar bone. The reason for performing this step prior to adaptation of the biomaterials to the defect is twofold: The periosteal releasing incision will typically initiate increased bleeding immediately following this step, which will complicate graft and membrane handling if completed after adaptation of the biomaterials. Also, because stabilization with sutures is dependent on using the apical periosteum as an anchor, performing the periosteal release following stabilization will not be possible without losing tension in the stabilization sutures and can lead to over-thinning of the buccal flap and even flap perforation.
Once adequate release in the flap has been achieved, stabilization can be initiated. A composite graft consisting of allograft and autogenous bone scrapings is applied to the defect and a resorbable collagen membrane can be adapted to confine the bone graft to the defect. The membrane must extend at least 3 mm to 5 mm beyond the margins of the defect. Applying modest pressure to the graft and overlying membrane with saline-saturated sterile gauze is recommended at this time to achieve initial stability of the complex by conforming the membrane over the graft, which facilitates stabilization of the complex with sutures.
If only one vertical releasing incision is being used, the authors recommend initiating stabilization at the site furthest from this incision (Figure 1). This will allow easier access to the periosteum for further suturing. If the first periosteal bite is close to the vertical incision, access to the apical periosteum at the other end of the membrane will be challenging.
To begin stabilization, a single bite with the suture needle is made into the periosteum apical to the periosteal releasing incision 3 mm to 5 mm lateral to the margins of the defect. The suture is then laid over the membrane and will enter the palatal or lingual flap from the internal to external direction, 5 mm to 7 mm apical to the flap margin. The operator must pull the suture slowly and precisely to keep the suture over the adapted membrane. The suture re-enters the palatal or lingual flap 3 mm to 5 mm lateral to the previous bite at the same apicocoronal level, passing back through the palatal flap. At this time the suture is pulled taut, and the stabilizing suture can be tied off with modest pressure. Ideally, the stabilization sutures are positioned over the collagen membrane, overlying native bone beyond the margins of the defect as opposed to over particulate graft material, which would have the potential to compress the particulate graft material and limit the space-maintaining potential of the technique.
Additional stabilization sutures can then be placed as needed to confine the graft and membrane to the defect. For mild horizontal ridge defects within the contour of the ridge, a single periosteal suture may provide adequate stability. For long-span defects and defects outside the alveolar housing, additional stabilization sutures are recommended. In this scenario, after the placement of the initial stabilization suture, additional graft material may be applied to the defect and the graft material can be further adapted to improve the space maintenance within the defect prior to placement of additional stabilization sutures in the same manner as described above.
Once adequate stabilization has been achieved, the buccal and palatal or lingual flap should be able to lay passively over the site with no loss of tension in the stabilization sutures. Thus, it is important to note that while making bites into the palatal or lingual tissues, the flap should be repositioned and held coronally to ensure that the stabilization suture will stay taut during eventual primary closure. It is also important that the bite of the palatal or lingual flap is more than 5 mm to 7 mm from the flap margin to ensure that the dual-layer closure will still be possible, with an ideal connective tissue-to-connective tissue interface of 5 mm to 7 mm.
In some instances, initiating the stabilization sutures through the palatal or lingual flap may be beneficial. The suture will then be laid over the membrane and made to bite the apical periosteum on the buccal side, and then, importantly, back through the palatal or lingual flap 3 mm to 5 mm away from the initial bite. In this situation the suture can then be tied off and the knot will be on the palatal or lingual surface as opposed to underneath the flaps, allowing modest pressure to be applied over the defect. In addition, this allows the sutures to be guided and placed over the membrane very precisely.
The following two cases demonstate the use of the Ribroast technique for horizontal guided bone regeneration.
Case 1
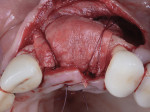
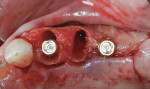
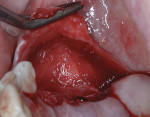
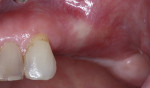
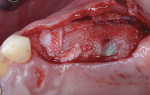
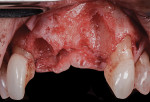
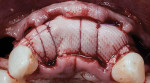
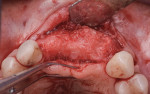
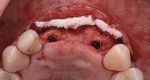
A 55-year-old female patient with no contributory medical history presented for replacement of her maxillary left first premolar and first molar with an implant-supported fixed restoration. The first molar had been extracted 10 years prior and the site presented with a moderate ridge width deficiency. Following administration of local anesthesia, a 15c blade was used to place a mid-crestal incision in the edentulous span. After full-thickness flap reflection, the maxillary left first and second premolars were extracted, implant osteotomies were performed, and implants (Tapered Plus bone level, BioHorizons, biohorizons.com) were placed in prosthetically driven positions at the first premolar and first molar sites. A residual buccal plate of less than 1 mm at the site of the first molar implant was observed (Figure 2). Periosteal scoring and debundling was then completed to release flap tension and provide access to the apical periosteum for stabilization sutures as described above (Figure 3).
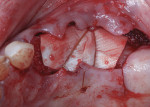
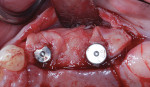
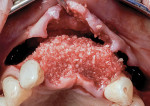
Autogenous bone collected from the adjacent site with a disposable bone scraper (Safescraper® TWIST, Geistlich Pharma, geistlich-na.com) was combined with a freeze-dried bone allograft (MinerOss® Cortical and Cancellous, BioHorizons) in a 1:1 ratio and adapted to the buccal plate defect at the first molar site and to the residual sockets of the first and second premolars. A crosslinked collagen membrane (OSSIX® Plus, Datum Dental Ltd., datumdental.com) was adapted over the ridge, and a single periosteal biting stabilization suture was placed as described above (Figure 4). Primary closure was then obtained by placing two horizontal mattress sutures 7 mm to 10 mm apart followed by multiple single interrupted sutures every 3 mm to 5 mm along the length of the flap.
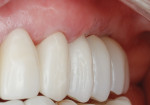
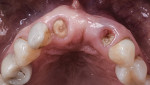
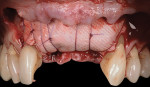
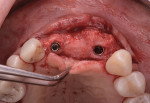
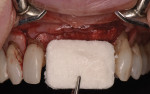
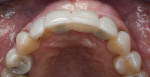
The patient was seen at 2 weeks, 2 months, and 4 months post-surgery and showed no signs of pain, swelling, or infection. At 5 months post-surgery the patient presented for uncovery (Figure 5). Due to displacement of the MGJ in the coronal direction, a palatal displaced crestal incision was performed and a full-thickness flap was reflected to expose the healed ridge (Figure 6). Complete defect fill was noted and a significant increase in the ridge width buccal to the first molar implant was observed. After placement of 3-mm tall healing abutments, a connective tissue graft was harvested from the internal aspect of the palatal flap and secured to the buccal side of the implants to increase soft-tissue quality and quantity (Figure 7). The implants were subsequently restored with a screw-retained implant-supported ceramic bridge (Figure 8).
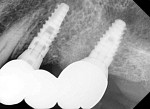
Figure 9 shows a periapical radiograph at 2 years post-crown delivery demonstrating stable bone levels following rehabilitation with an implant-supported bridge.
Case 2
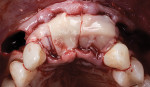
A 75-year-old female patient was referred for rehabilitation with dental implants following the fracture and loss of a three-unit fixed prosthesis at the site of the maxillary right central and left lateral incisors (Figure 10). Both teeth had lost all coronal tooth structure and were deemed nonrestorable. The left lateral incisor was found to have a 7 mm facial pocket depth with complete loss of the labial plate, as noted on the CBCT. A treatment plan involving replacement of the fractured teeth with dental implants to support a three-unit implant-supported fixed prosthesis was agreed to.
After administration of sufficient local anesthesia, an intrasulcular incision was placed, with a mid-crestal incision at the right central incisor site and vertical releasing incisions distal to the right lateral incisor and left cuspid. A full-thickness flap was elevated, the maxillary right central incisor and left lateral incisor were extracted, and the left lateral incisor site was thoroughly degranulated until the full extent of the wide labial defect extending to the apex of the socket could be visualized (Figure 11). The facial plate of the right central incisor was present with approximately 1 mm in thickness and had a vertical fracture line along the mesial aspect. Autogenous cortical shavings were obtained from the facial alveolar ridge adjacent to the recipient site using a bone scraper (Safescraper TWIST) and combined with a cortico-cancellous mineralized allograft (OraGraft® Cortical/Cancellous Mineralized Particulate 50/50 Mix, Lifenet Health, lifenethealth.org) in approximately a 1:3 ratio, along with leukocyte- and platelet-rich fibrin (L-PRF) serum for the purpose of improving the graft's handling properties.
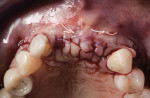
A new 15c scalpel blade was used to make a 1-mm periosteal incision toward the apical area of the labial flap, and a microsurgical periosteal elevator (Mini Me®, Hu-Friedy, hufriedygroup.com) was used to stretch the flap from the incision line in the incisal direction to create sufficient flap release. The composite particulate graft was then placed into the sockets and on the facial aspect of the alveolar ridge, with significant overgrafting or overbuilding of the buccal contour (Figure 12). After hydration with normal saline, a crosslinked collagen membrane was trimmed and adapted to cover the entirety of the graft, on both facial and occlusal aspects.
The periosteal sutures were placed in the following manner: A 6-0 suture was used to bite the base of the apical periosteum (the portion that was separated from the labial flap) at the right central incisor area. The suture was then laid over the membrane, entering the palatal flap from the periosteal side. The suture was then passed through the epithelial side of the palatal flap 5 mm to 7 mm medially and was tied with the knot laying on the facial aspect of the membrane. This was repeated over the left central incisor site before additional graft was placed on the facial aspect of the left lateral incisor. The periosteal biting suture was then repeated, holding the membrane firmly down on the mesial side of the left cuspid, whereas the re-entry on the palatal aspect was placed distal to the left cuspid. This allowed for the membrane to remain in contact with the native ridge and would prevent particulate graft from migrating distally. The same protocol was repeated around the right lateral incisor (Figure 13 and Figure 14).
Once stability of the graft-membrane complex was verified, L-PRF membranes (IntraSpin®, Intra-Lock, BioHorizons) were placed over the resorbable collagen membrane and specifically under the incision line (Figure 15). Two 3-0 dense-polytetrafluoroethylene (dPTFE) horizontal mattress sutures were placed to relieve the flap tension at the incision line. Simple 6-0 interrupted sutures were then placed to close the crestal and vertical incisions, allowing for tension-free primary closure (Figure 16).
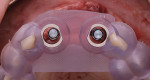
After 7 months of healing, the site was re-entered and excellent bone regeneration was noted (Figure 17). Implants (Straumann® BLT, Straumann, straumann.com) were placed into the planned restoratively driven positions using a 3D-printed surgical guide (Figure 18 and Figure 19). A volume-stable collagen matrix (Fibro-Gide®, Geistlich Pharma) was trimmed and placed under the labial flap to thicken the gingival phenotype (Figure 20 and Figure 21). After a healing period of 3 months, a three-unit fixed implant-supported prosthesis was fabricated and delivered (Figure 22).
Discussion
Stabilization of the graft-membrane complex is needed for optimal regeneration to occur.4 When the use of screws or pins is contraindicated due to the fragility of a resorbable membrane, difficult access for placement of tacks or screws, or where the use of fixation screws and pins is otherwise less than ideal, periosteal biting sutures may be beneficial.
The advantage of placing multiple individual horizontal mattress sutures over a single continuous suture is twofold. Placement and securing of a stabilizing suture at one end of the defect allows the clinician to then add additional bone graft materials and adapt the graft material to precisely fill the defect before placing additional stabilizing sutures. Moreover, individual periosteal sutures are easier to place and more forgiving than a continuous suture that has the potential to loosen, resulting in a complete loss of stabilization. Tension of individual sutures over the membrane can be easily modified, whereas once a continuous suture is placed, adjustment of one area modifies the tension over the entire complex. The number of individual stabilization sutures needed will depend on the anatomy and characteristics of the defect being grafted and the regenerative needs of the case. Use of bone graft materials with improved handling is advantageous. For instance, "sticky" bone provides a graft that will adhere to the defect and be easier to work with.20
While mild vertical regeneration can be achieved with this technique, the authors recommend the use of nonresorbable membranes such as titanium-reinforced PTFE (ti-PTFE),8 ti-mesh,21 or Khoury bone plates22 when success is dependent on the regeneration of significant alveolar bone height. In addition, the use of stabilization sutures is not recommended for the stabilization of ti-mesh and ti-PTFE membranes because complete fixation of these materials is necessary beyond the resorption time of the sutures.
Conclusion
With the Ribroast technique, individual periosteal biting horizontal mattress stabilization sutures are used to secure a graft-membrane complex to achieve ridge augmentation. The use of periosteal biting stabilization sutures may be a viable option for the regeneration of ridge width defects as opposed to tacks and screws. Sites that present with concave defects and defects within the housing of the predetermined genetic skeletal envelope are good candidates for this technique. In addition, this technique may be used in combination with tenting screws to improve space maintenance for defects that require bone regeneration beyond the alveolar contour, but more research is needed to identify the severity of defects that can be treated predictably with this technique.
About the Authors
Matthew Fien, DDS
Co-Medical Director, International Dentistry Research Group; Private Practice,
Fort Lauderdale, Florida
Israel Puterman, DMD, MSD
Co-Medical Director, International Dentistry Research Group; Private Practice Chevy Chase, Maryland
Juan Mesquida, DDS
Co-Medical Director, International Dentistry Research Group; Private Practice, Palma de Mallorca, Spain
Guillermo Bauza, PhD
Co-founder, Research Director, International Dentistry Research Group; Faculty of Medicine, Health and Life Science, Swansea University, Swansea, Wales;
Private Practice, Palma de Mallorca, Spain
Ignacio Ginebreda, DDS
Private Practice, Barcelona, Spain
References
1. Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81(5):672-676.
2. Burchardt H. Biology of bone transplantation. Orthop Clin North Am. 1987;18(2):187-196.
3. Buser D. 20 Years of Guided Bone Regeneration in Implant Dentistry. 2nd ed. Chicago, IL: Quintessence Publishing; 2009:272.
4. Wang HL, Boyapati L. "PASS" principles for predictable bone regeneration. Implant Dent. 2006;15(1):8-17.
5. Epari DR, Schell H, Bail HJ, Duda GN. Instability prolongs the chondral phase during bone healing in sheep. Bone. 2006;38(6):864-870.
6. Wazen RM, Currey JA, Guo H, et al. Micromotion-induced strain fields influence early stages of repair at bone-implant interfaces. Acta Biomater. 2013;9(5):6663-6674.
7. Irandoust S, Müftü S. The interplay between bone healing and remodeling around dental implants. Sci Rep. 2020;10(1):4335.
8. Urban IA, Jovanovic SA, Lozada JL. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: a retrospective study of 35 patients 12 to 72 months after loading. Int J Oral Maxillofac Implants. 2009;24(3):502-510.
9. Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001;72(4):512-516.
10. Urban IA, Nagursky H, Lozada JL. Horizontal ridge augmentation with a resorbable membrane and particulated autogenous bone with or without anorganic bovine bone-derived mineral: a prospective case series in 22 patients. Int J Oral Maxillofac Implants. 2011;26(2)404-414.
11. Buser D, Dula K, Belser UC, et al. Localized ridge augmentation using guided bone regeneration. II. Surgical procedure in the mandible. Int J Periodontics Restorative Dent. 1995;15(1):10-29.
12. Steigmann M, Salama M, Wang HL. Periosteal pocket flap for horizontal bone regeneration: a case series. Int J Periodontics Restorative Dent. 2012;32(3):311-320.
13. Romanos GE. Periosteal releasing incision for successful coverage of augmented sites. A technical note. J Oral Implantol. 2010;36(1):25-30.
14. Urban IA, Lozada JL, Wessing B, et al. Vertical bone grafting and periosteal vertical mattress suture for the fixation of resorbable membranes and stabilization of particulate grafts in horizontal guided bone regeneration to achieve more predictable results: a technical report. Int J Periodontics Restorative Dent. 2016;36(2):153-159.
15. Neiva R, Duarte W, Tanello B, Silva F. LASSO GBR-rationale, technique, and long-term results. Clin Oral Implants Res. 2018;29(S17):447.
16. Neiva BT, Neiva GF, Neiva R. Lasso guided bone regeneration technique for the management of implant fenestration defects. Compend Contin Educ Dent. 2022;43(7):426-432.
17. Greenstein G, Greenstein B, Cavallaro J, Tarnow D. The role of bone decortication in enhancing the results of guided bone regeneration: a literature review. J Periodontol. 2009;80(2):175-189.
18. Ronda M, Stacchi C. A novel approach for the coronal advancement of the buccal flap. Int J Periodontics Restorative Dent. 2015;35(6):795-801.
19. Pasqualini D, Cocero N, Castella A, et al. Primary and secondary closure of the surgical wound after removal of impacted mandibular third molars: a comparative study. Int J Oral Maxillofac Surg. 2005;34(1):52-57.
20. Sohn DS, Huang B, Kim J, et al. Utilization of autologous concentrated growth factors (CGF) enriched bone graft matrix (sticky bone) and CGF-enriched fibrin membrane in implant dentistry. J Implant Adv Clin Dent. 2015;7(10):11-29.
21. von Arx T, Hardt N, Wallkamm B. The TIME technique: a new method for localized alveolar ridge augmentation prior to placement of dental implants. Int J Oral Maxillofac Implants. 1996;11(3):387-394.
22. Khoury F, Khoury C. Mandibular bone block grafts: diagnosis, instrumentation, harvesting techniques and surgical procedures. In: Khoury F, Antoun H, Missika P, eds. Bone Augmentation in Oral Implantology. Berlin: Quintessence Publishing; 2007.